-
-
Building 5,18 Yueluo Road, Yuepu Town, Baoshan District, Shanghai, China
Call Anytime
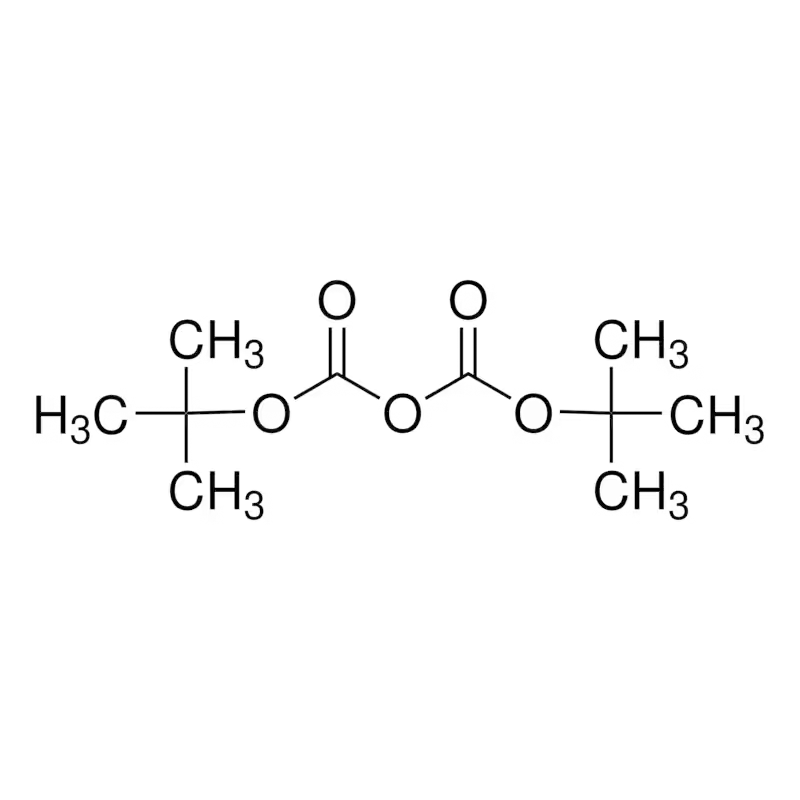
+86 131 2053 3622Di-tert-butyl dicarbonate Cas 24424-99-5 BOC

Key attributes
Industry-specific attributes
CAS No. : 24424-99-5
Purity: 99%
Other attributes
Place of Origin: Shandong, China
Type: Agrochemical Intermediates, Dyestuff Intermediates, Flavor & Fragrance Intermediates, Syntheses Material Intermediates
Other Names: BOC
MF: C10H18O5
EINECS No.: 246-240-1
Brand Name: Haoyi Shuoda
Model Number: BOC
Application: amidogen protective agent
Appearance: Colorless liquid or white crystal
introduction
Di-tert-butyl dicarbonate (Boc2O) is a widely used reagent for introducing protecting groups in peptide synthesis. It plays an important role in the preparation of 6-acetyl-1,2,3,4-tetrahydropyridine by reacting with 2-piperidone. It serves as a protecting group used in solid phase peptide synthesis.
BOC acid anhydride is an organic compound with the molecular formula C10H18O5. It is used for introducing the protective group of tert-butoxycarboxyl in organic synthesis, especially for all the amino acid head amino group
Application
Di-tert-butyl dicarbonate is a carboxylate derivative, which can be widely used in the synthesis of medicine, protein and polypeptide, biochemical food, cosmetics and other products. It is used as an intermediate in medicine and organic synthesis. It is an important amino protective agent used in the synthesis of medicine and agrochemical products. Used in organic synthesis to introduce tert-butoxycarbonyl protective groups. Di-tert-butyl dicarbonate (Boc anhydride) is used in organic synthesis to introduce tert-butoxycarbonyl protective genes, especially for amino protection of amino acids. Widely used in medicine, protein and peptide synthesis, biochemical food, cosmetics and other products synthesis. Amino acid BOC reagent. Used in organic synthesis to introduce tert-butoxycarbonyl (BOC) protection groups, especially for amino protection of amino acids. Reagent for the preparation of tert-butoxycarbonyl protected amines. A reagent for introducing a tert-butoxycarbonyl protective group. Alcohols such as tert-butoxycarbonyl derivatives can be protected by Lewis acid catalysis. The synthesis of tert-butoxycarbonyl aminopropyl with formaldehyde, diisopropylamine, and copper bromide The reaction is used to prepare aminomethylallylene, a component of many bovine plasma amine oxidase inactivators.