-
-
Building 5,18 Yueluo Road, Yuepu Town, Baoshan District, Shanghai, China
Call Anytime
+86 131 2053 36224-tert-butoxystyrene,CAS No:95418-58-9
Basic information of 4-tert-butoxystyrene:
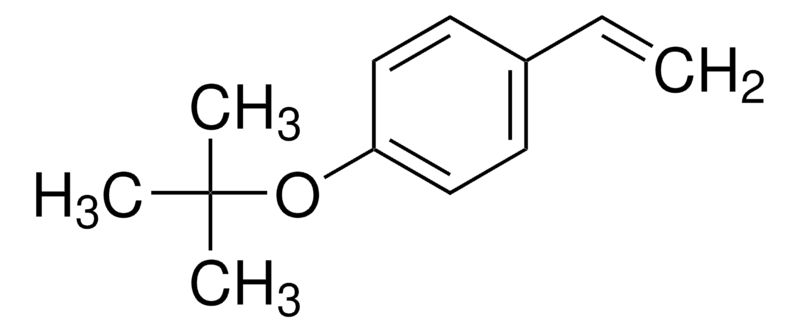
Name: 4-tert-Butoxystyrene
Synonymous name:
P-T-BUTOXYSTYRENE;4-TERT-BUTOXYSTYRENE;1-Vinyl-4-(tert-butyloxy)benzene;4-(tert-Butyloxy)styrene;4-tChemicalbookert-Butyloxystyrene;p-(tert-Butyloxy)styrene;tert-Butyl(4-vinylphenyl)ether;(1-Butoxyethenyl)benzene
CAS No:95418-58-9
Molecular formula: C12H16O
Molecular weight: 176.25
EINECS No:619-127-3
Properties of 4-tert-butoxystyrene:
Melting point: −38 °C(lit.)
Boiling point: 256.8±9.0 °C
Density: 0.936 g/mL at 25 °C(lit.)
Vapor pressure: 17.999Pa at 20℃
Refractive index: n20/D 1.524(lit.)
Flash point: 207 oF
Storage conditions: 2-8℃
InChI:InChI=1S/C12H16O/c1-5-10-6-8-11(9-7-10)13-12(2,3)4/h5-9H,1H2,2-4H3
InChIKey:GRFNSWBVXHLTCI-UHFFFAOYSA-N
SMILES:C1(OC(C)(C)C)=CC=C(C=C)C=C1
LogP:3.83 at 22℃ and pH7
CAS Database:95418-58-9(CAS DataBase Reference)
The Uses and Synthesis Methods of 4-tert-Butoxystyrene
Chemical Properties
4-tert-Butoxystyrene is an electron-rich styrene compound exhibiting exceptionally high chemical reactivity. The double bond within its structure can undergo epoxidation reactions in the presence of oxidizing agents and can also engage in electrophilic addition reactions with hydrogen halides. According to relevant literature, this compound can participate in Heck coupling reactions with aryl halides under the catalysis of palladium-based metal catalysts, yielding 1,2-disubstituted alkene derivatives.
Purpose
4-tert-Butoxystyrene serves as a critical intermediate in organic synthesis and functions as a fundamental chemical raw material in the daily chemical industry. In organic chemistry research, it facilitates the preparation of functionalized phenylethane derivatives due to the high chemical conversion activity of its double bond unit. Additionally, given its distinctive sweet and fragrant aroma, 4-tert-butoxystyrene finds application in the industrial production of daily chemical products such as soap and shampoo.